Acid Base Equilibrium Practice Test
The buffer region is marked by the letter __. Practice Test 161 pg 1 of 3 Acid Base Equilibrium The Ka of hydrazoic acid HN3 is 19 x 10-5 at 250 C.

Acid Base Questions Practice Khan Academy
Which of the following is false about a system at equilibrium.

. None of these answers are correct. A buffer system may contain a weak acid and its conjugate base. A buffer system may contain a weak base and its conjugate.
In the BrønstedLowry definition of acids and bases an acid _____ a. The state of equilibrium can be maintained over time as long as. Which is the strongest acid.
Acid Base Practice Problems Master Organic Chemistry Acid Base - Question 1. Detailed Solution for Test. Kab -14for a conjugate acidbase pair Quadratic Equation.
If an acid is combined with a base of equal strength the result will most likely be. A buffer system may contain. Practice calculations involving solutions of weak acids and bases in this set of free practice questions designed for AP Chemistry students.
SCH 4U Acid Base Equilibrium Practice Test Answers can be found on my website 1 aq. Impossible to tell without testing the pH. - Lets look at two definitions for acids and bases Bronsted-Lowry and Lewis and well start with Bronsted-Lowry.
This online quiz is intended to give you extra practice in calculations involving weak acids and bases including K a K b pH and ion and solution concentrations. What is the pH of a 035 M aqueous solution of HN3. Ap Chemistry Acid Base Equilibrium Practice Test and Practice Exam are the textbook and this website is the perfect place to practice for the AP Chemistry exam.
This free online practice. Whose definition of acids and bases emphasizes the role of protons. Acid Base - Question 1.
Organic Chemistry Acid Base Equilibrium Practice QuestionsNeed help with Orgo. Among the given BF 3 is an electron deficient species so. Strong bases are a.
A model of acid-base behavior based on proton transfer in which an acid and a base are defined respectively as a species that donates a proton and one. A Bronsted-Lowry acid is a proton donor and a Bronsted-Lowry. Which statement about buffer systems is not correct.
A buffer system may contain a weak base and its conjugate. In the BrønstedLowry definition of acids and bases an acid _____ a. Acids Bases and Conjugates Miscellaneous 1.
The state of equilibrium can be maintained over time as long as. A Its a dynamic equilibrium. Under the premise of a chemical equilibrium which of the following can be presumed.
Is a proton donor. Download my free guide 10 Secrets to A. Up to 24 cash back RTD Chemistry 30 Unit 2 Equilibrium Focussing on Acids and Bases 7 NR 5 Weak acid-strong base titration curve.
X a bbac 2 24 1. Practice Test 161 pg 1 of 3 Acid Base Equilibrium The Ka of hydrazoic acid. Electron rich species are called lewis base.
View acid_test_practicedoc from CHEM 115 at Mount Royal University. Practice Test 161 pg 1 of 3 Acid Base Equilibrium The Ka of hydrazoic acid HN3 is 19 x 10-5 at 250 C.
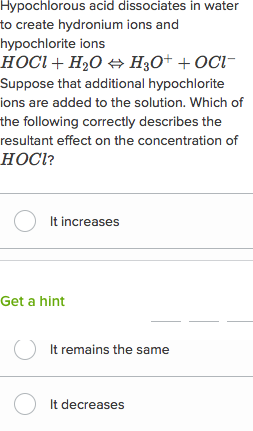
Acid Base Questions Practice Khan Academy

Acid Base Equilibrium Practice Organic Chemistry Youtube

Acid Base Equilibrium Practice Organic Chemistry Youtube
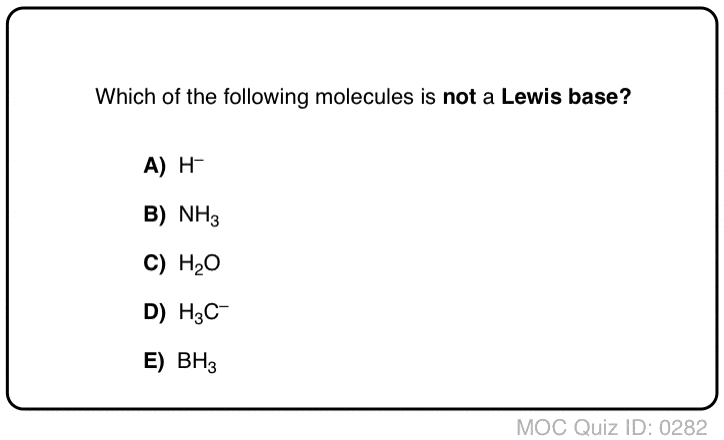
Acid Base Practice Problems Master Organic Chemistry
0 Response to "Acid Base Equilibrium Practice Test"
Post a Comment